Our 21 CFR Part 11 compliance based automated process control system is designed specifically for pharmaceutical processing equipment.
Our SCADA based control systems are designed to meet the precise hardware and software requirements of the customer using disciplined and documented procedures. The control design system is based on proven process technology to control process parameter, system monitoring, alarming, feedback, and data acquisition. Validation documentation and field verification services are available for all control systems.
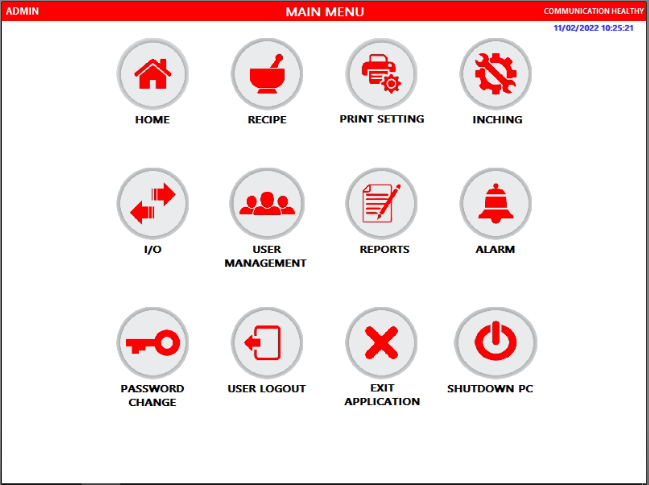
We offer below features under this panel:
Operator-Friendly Interface
- Automatic (Recipe-Driven) and Manual Modes
- Customised to suit country / customer specific language
- Built with Industrial duty HMI (IPC) with touch screen
- Print out after completion of every cycle
Regulatory compliance
- Electronic Management for All Data and Records: Recipes, Batch Reports, Alarms, Security Files
- Data collection and reporting for mixer specifications – speed, time, direction of rotation
- Data back up facility available – During power failure and During life cycle
Recipe Management
- Unlimited Number of Recipes creation
- Can have option of multiple cycles in single recipe
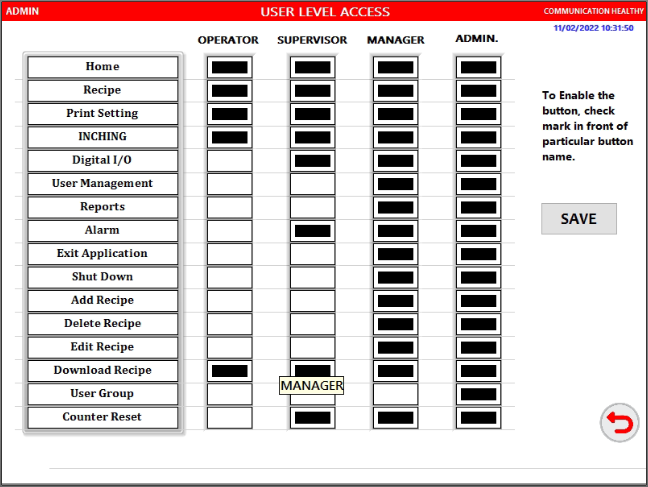
Configurable Security System
- Individual Login Names and Passwords with Password Expiration Control. Four level of controls are offered – Administrator, Manager, Supervisor and Operator
- Auto log off, Minimum password length with Alpha Numeric combination, Masked password entry
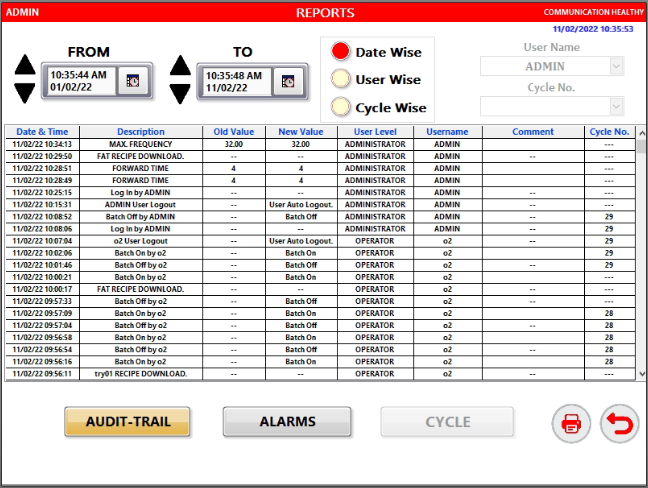
- Audit trail report with Alarm listing and history record